Abstract
Background: Quantitative Sensory Testing (QST) is used in a variety of pain disorders such as arthritis and neuropathic pain syndromes, to characterize pain and predict prognosis and response to specific therapies. QST evaluates sensory function using a set of psychophysical procedures to measure subjective somatosensory responses to various physical stimuli. In Sickle Cell Disease (SCD) the painful vasoocclusive crisis is the most difficult management challenge and factors underlying the transition from acute to chronic pain and the wide interpatient variability in the pain experience are poorly understood. Optimal management for this complex pain syndrome remains unclear despite over three decades of basic and clinical research in the field.In this study, we aimed to duplicate results in the literature documenting altered QST thresholds in SCD and test the reproducibility of results over time to validate QST as a therapeutic endpoint for use in clinical trials evaluating novel preventative pain therapies.
Objectives: Primary objectives were to test thermal thresholds in children with SCD versus African American controls and determine consistency over 6 months. Exploratory objectives were to correlate QST with clinical factors and select biomarkers and assess pressure pain thresholds (PPTs).
Methods: Fifty-seven SCD and 60 control subjects aged 8-20 years underwent heat (wdt) and cold (cdt) detection and pain (hpt and cpt) threshold testing using a Medoc TSAII. A thermal composite score (TCS) was constructed by averaging the wdt, cdt, hpt, and cpt threshold responses from the testing instrument's starting temperature of 32°C. All participants were tested at baseline and 3 months; SCD subjects were additionally tested at 6 months and blood collected for future biomarker assessment. PPTs were performed using an SBMedic algometer. A single individual performed all QST. Descriptive statistics were computed and covariate-adjusted differences in thresholds within and between groups evaluated over time by mixed effects regression models with random intercepts and linear contrast tests. The intraclass correlation coefficient (ICC) from these models was used to assess within-subject consistency and Spearman's correlation was used to evaluate associations between thresholds and biomarkers. In addition, as a nested pseudo-intervention study, we analyzed a subset of "new" hydroxyurea (HU) users consisting of SCD subjects in whom HU was initiated within a few weeks prior to, or shortly after baseline testing. Significance was set at 0.05.
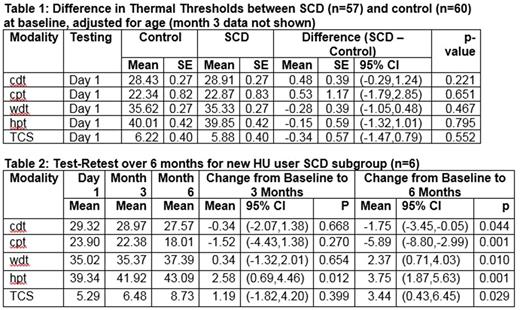
Results: There were no significant differences in mean thermal or PPTs between SCD and control participants at baseline (Table 1) or month 3. QST measurements were consistent in SCD and control subjects for cpt, wdt, hpt and TCS (each ICC>0.55), but not necessarily for cdt (ICC = 0.37). Although generally internally consistent, there was some variability in mean thermal thresholds among SCD subjects over time, but the only significant difference between the 3 and 6 month means was for hpt (+0.67°C; 95% CI 1.15, 0.19). There was no association between mean thermal thresholds and chronic transfusion therapy. The group in whom HU was initiated shortly after baseline testing (n=6) demonstrated significant reductions in all thermal sensitivity and pain threshold measurements following HU initiation (Table 2).
Conclusions: In contrast to Brandow's study (Brandow, American Journal Hematology, 2012), which documented thermal hypersensitivity in SCD, we have not demonstrated significant differences in thermal thresholds between SCD and control subjects. This could be due, in part, to differences in body sites used for testing. Results between second and third test sessions were highly consistent, indicating that reliability may improve once subjects have experience with QST. Subjects on whom HU was initiated shortly after baseline testing (new HU users) exhibited progressive and significant decreases in both cold and heat sensitivity from baseline to 6 months, suggesting that thermal testing is sensitive to effective therapy to prevent vasoocclusive pain. These findings are of critical importance to inform the planning of future SCD related clinical trials using QST as an endpoint in the evaluation of preventative pain therapies.
Supported by National Institute of General Medical Sciences National Institutes of Health Award P20GM109021 and U54GM104941
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal